Electric batteries are an electric device that gives power to an electrical device. It consists of two or more electrochemical cells. In history, it was known as a device consisting of multiple cells, and is usable for devices consisting of a single cell. These cells are connected to an external source and provide a power supply due to the flow of electrons through two terminals known as the cathode (positive charge) and anode (negative charge).
A redox reaction is produced when the battery is connected to the external load and electrons flow towards the cation, attracting positive charges. The reactants having higher energies are converted into lower energy, and extra energy is provided to the circuit as electric power.
Electric batteries are available in multiple sizes, from miniature cells used in wristwatches to huge battery banks with room-sized cells used in computer data centers.

History
The term battery was first described by Benjamin Franklin when he was working on the Leyden jar capacitors in 1749. The voltaic pile was first designed by Alessandro Volta in 1800 and composed of zinc and copper plates separated by brine-soaked paper disks. It produces a steady current, transforming to a considerable length in the proper way. However, Michael Faraday invented an electric battery in 1834 with a fluctuating voltage, which does not allow for a larger current for a longer time.
The industrial-level and electrical telegraph network power supply was considered the Daniel cell introduced by John Frederic Daniel in 1836. The Daniel cell is composed of a copper sulfate solution with a copper pot within an earthenware container filled with zinc electrodes and sulfuric acid. This liquid cell caused leakage, and it is covered with a glass jar but still very dangerous, which is why it is unsuitable for appliances.
Principles of Electric Batteries
The basic principle of a battery is to convert chemical energy to electrical energy through electrochemical reactions. This electrical energy is released as the bond energies or the cohesive energies of the metals, oxides, and molecules that are involved in the electrochemical reactions.
Unlike transition metals, zinc and lithium are high-energy metals due to the deficiency of transition electrons, and energy can easily be stored in these metals. When electrons move in the external circuits, then redox reactions occur energetically.
Electric batteries consist of multiple numbers of voltaic cells, and each cell is composed of two half cells, each connected in series, having electrolytes with positive metal ions. The half-cell on which an anion or negative charge migrates is known as the negative electrode having electrolyte, but the other part of the cell where a positive ion migrates is called the positive electrode with electrolyte.
When a metal oxidizes, an electron is released from an anion and moves to the positive electrode, and electric energy is generated due to the motion of electrons. Some cells use different electrolytes to operate the electric circuit completely instead of using any other separator. Any liquid or moist substance having ions that can flow electrons can be used as an electrolyte.
EMF (ElectroMotive Force) of electric batteries
Every single part of the cell has EMF, which is usually measured in volts. The total EMF of a cell is the difference between its two cell EMFs. Such as:
En = E2 – E1
Where En is the net EMF.
E2 is the emf of the second half-cell.
E1 is the emf of the first half of the cell.
How does battery voltage work?
The voltage is produced in the electric batteries due to the chemical reactions between the electrolyte and electrodes. The driving force across the cells is known as the terminal voltage, measured in volts. When a battery is just sitting unused, meaning it’s not charging and discharging, its voltage is called the open-circuit voltage, which is almost equal to the electric batteries EMF (electromotive force). When a cell is charging, it exceeds the terminal voltage and then the open circuit voltage, but when it is discharging, it has more open circuit voltage as compared to the terminal voltage due to the internal resistance.
Perfect battery
A perfect cell has zero resistance so that it can maintain the terminal voltage consistently until it gets discharged. For example, if a cell has 1.5 volts, then it will perform work of 1.5 joules and make a charge of one coulomb until it drops to zero.
Real battery
In the real battery, the internal resistance increases, and the open-circuit voltage decreases during discharge. If we make a graph between resistance or voltage and time, then we will get a curve. When we change the arrangement of the circuit and chemistry, then the shape of the curve will change.
Types of battery
There are two types of electric batteries:
1. Primary Battery
These batteries are not rechargeable, and users can use them until their energy is exhausted. When the supply of reactants is exhausted, then the current stops, and it becomes useless because it does not generate any chemical reactions to get charged. These types of batteries are used in alarms, telegraphs, and communication circuits; due to the low current drain, they are not used in portable devices.
Alkaline and zinc-carbon batteries are disposable primary batteries having very high densities, but they are not used in high-drain applications under 75 ohms. But don’t try to recharge the disposable primary batteries because they have irreversible reactions and cause major damage.
2. Secondary Battery
The rechargeable electric batteries are known as secondary batteries because when they are connected to an electric current, they generate reversible chemical reactions. The chemical reactants help in recharging the cell multiple times. If they have low electrolytes, less internal corrosion, and active metals that are rechargeable, then they definitely could not start charging.
When an electric current supply (charger) is given to these batteries, they start recharging. It is used in automobiles and boating. Lead-acid and car batteries are examples of rechargeable batteries.
Composition of electric batteries
With varying chemical processes, different electrochemicals are invented, such as voltaic piles, electrolytes, flow, fuel, and galvanic cells.
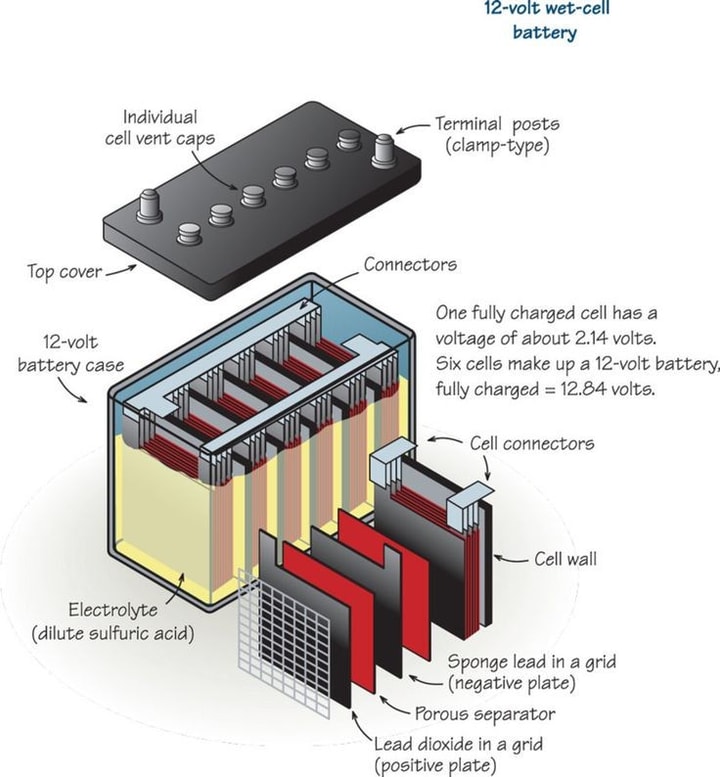
Wet cell
A wet cell is known as a vented or flooded cell in which liquid is filled in the internal part, and gases are produced that move to the air. Breakers very easily generate wet cells and are commonly used in tools to understand electrochemistry. Wet cells are primary cells that are not rechargeable and are used in industries for standby power, automobiles, uninterrupted power supplies, switch chargers, and telecommunications. Molten salt batteries are known as primary or secondary batteries that use molten salt as an electrolyte, are highly insulated, and operate at high temperatures.
Some wet cells of the electric batteries are given as
- Daniel cell
- Bunsen cell
- Grove cell
- Clark cell
- Chromic acid cell
- Leclanché cell
- Weston cell
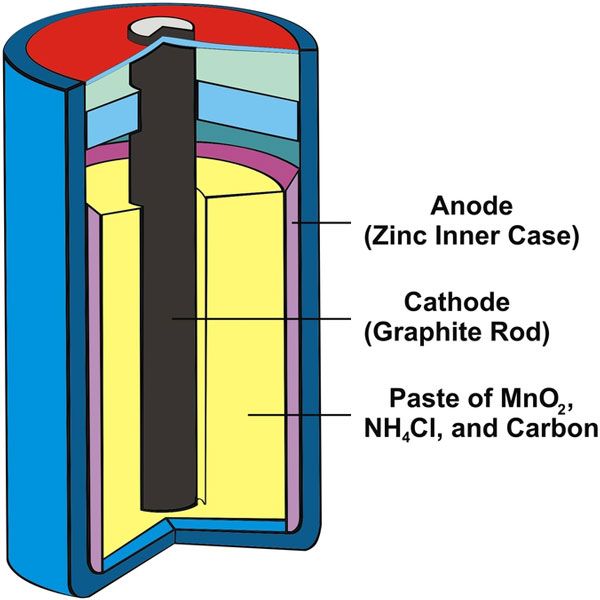
Dry cell
A dry cell is composed of paste electrolytes and is used for portable equipment. After the development of gel batteries, lead-acid batteries gained safety and portability. A standard dry cell is composed of zinc and carbon, where zinc works as an anode, carbon as a cathode, and aluminum chloride as the paste of electrolyte. The paste of magnesium dioxide and aluminum chloride is placed as the second electrolyte between the cathode carbon and the electrolyte.
Reserve batteries
Electric batteries can be stored for a longer time without any supply of power, and whenever it is needed, just charge them and start working. For example, by firing a gun, an electronic artillery fuze can be activated, accelerating the capsule of electrolytes and powering the fuze’s circuits. Reserve batteries are designed for a longer time with short service times, like some seconds or minutes. Water-activated batteries are examples of reserve batteries that are easily activated by water.
Performance, capacity, and discharge of the electric batteries
The characteristics of a battery depend upon the charge cycle, current drain, temperature, and internal chemistry. At low temperatures, it could not supply proper current as at higher temperatures. The equation of charge and discharge is given as
C = P1Q/a1
Here, C is the discharge of the cell.
P1Q is the total capacity of the cell.
A1 is the depletion of charge.
An electric charge supply at a voltage that does not drop the terminal voltage of the cell is known as a capacity measure in ampere-hours. A small cell having the same open circuit voltage and chemistry provides less capacity as compared to a larger one because it depends on the electrode material. When the discharge rate increases, capacity decreases, and Peukert’s law explains the relationship between discharge time, capacity, and the current.
t = Qp/Ik
Qp = Capacity of electric batteries
I = Drain current from battery
t = the sustain time of a battery
K = Constant (having value 1.3)
Inside, chemical reactions waste the charges of both rechargeable and disposable cells without any useful purpose. When cells are not used, they lose charges, and the process is known as self-discharge, mostly due to the side reactions that use the energy of the cell. The self-discharge depends upon the chemistry, the time it takes to discharge the charges, and the design. Whenever a cell is recharged, its lifetime decreases, and after many recharges, it dies.
The performance of the battery depends on the usage. If you are using it slowly, then energy is higher, and when you are using it fast, energy will be lost quickly. The C rate tells the charging speed; for example, if 1C is used in 1 hour, 0.5C is used in 2 hours. C rates are very important for the health of the cells.
The series connection in the battery
Electric batteries are connected in series-parallel ways or in a combination of both series and parallel. In a series, electrons travel in a single path, but in parallel connections have multiple paths. A battery is usually called a series battery when its one terminal is connected to the negative terminal of the next cell. The overall emf is the sum of the individual cells, such as:
E = E1 + E2 + E3 + E4…..+En
Same as the total internal resistance is equal to the individual cells’ resistance
r = r1 + r2 + r3 + r4 + …..+ rn
Advantage: The main advantage of the series battery is that it provides a high-voltage system with lower current, which helps you in using thinner wiring with very low voltage drop.
Disadvantage: But, for the series connection, you must use a converter for the low voltage.
Parallel connection in electric batteries
When all the positive terminals of the cells are connected and the negative terminals are connected, then they form a parallel connection. In the case of a parallel system, the emf of the whole system is equal to the emf of the individual cell, but the total current is equal to the sum of all the cells’ currents. The internal resistance is given as
(1/r1 + 1/r2 + 1/r3 + …..1/rn)^-1
Advantage: Its main advantage is that when a cell is not working properly, then others can work without any time wasted. But it can drop higher currents when connected with thicker wires.
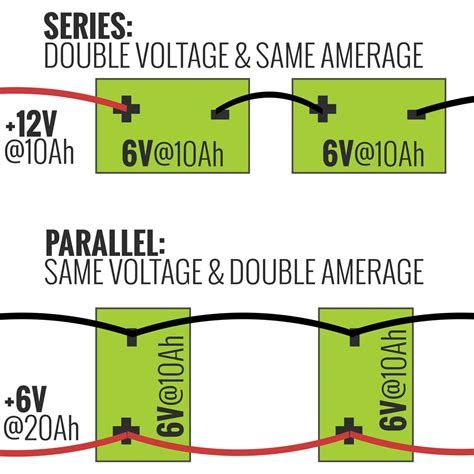
Series-parallel batteries
Some electric batteries have to complete both higher voltage and current; then they use series and parallel combinations. In this case, cells are connected in series, and then series are connected in a parallel way to complete the voltage and current as required.


